SERVICES
CMC and analytical development consulting meetings can be scheduled, as needed, or on a regular and recurring basis.
- Development Strategy
A well-thought-out CMC development strategy is essential to remaining competitive and meeting accelerated timelines. We have experience in bringing a highly complex small molecule such as Halaven, containing 19 stereogenic centers and made by a 62-step total synthesis, through clinical development and commercialization. We also have experience with large molecules, such as the 58 kD fusion protein, Ontak.
- Regulatory Feedback
Having clear, concise, well-written responses to regulatory inquiries helps ensure smooth development. We have extensive experience in answering questions from global regulatory authorities, and we advise and assist clients in this area.
- Problem Solving & Analytical Technology Issues
As structural complexity increases with development candidates and certain molecular features make production and analysis challenging, firms are likely to face analytical problems. We have experience developing analytical methods for complex molecules which pose interesting challenges.
CMO & CRO Management
Many biotech and pharmaceutical firms rely upon contract manufacturing organizations (CMOs) to support their CMC development and clinical supply activities. Identifying the right CMO and working with them, productively, has a major influence on smooth and successful CMC development. We have years of experience working with CMOs and CROs (contract research organizations). A sample of our typical services includes:
- Review of CMO & CRO Capabilities
- Participation in Regular Meetings
- Reviews of CMC Reports
- Problem Solving
Document QC Reviewing
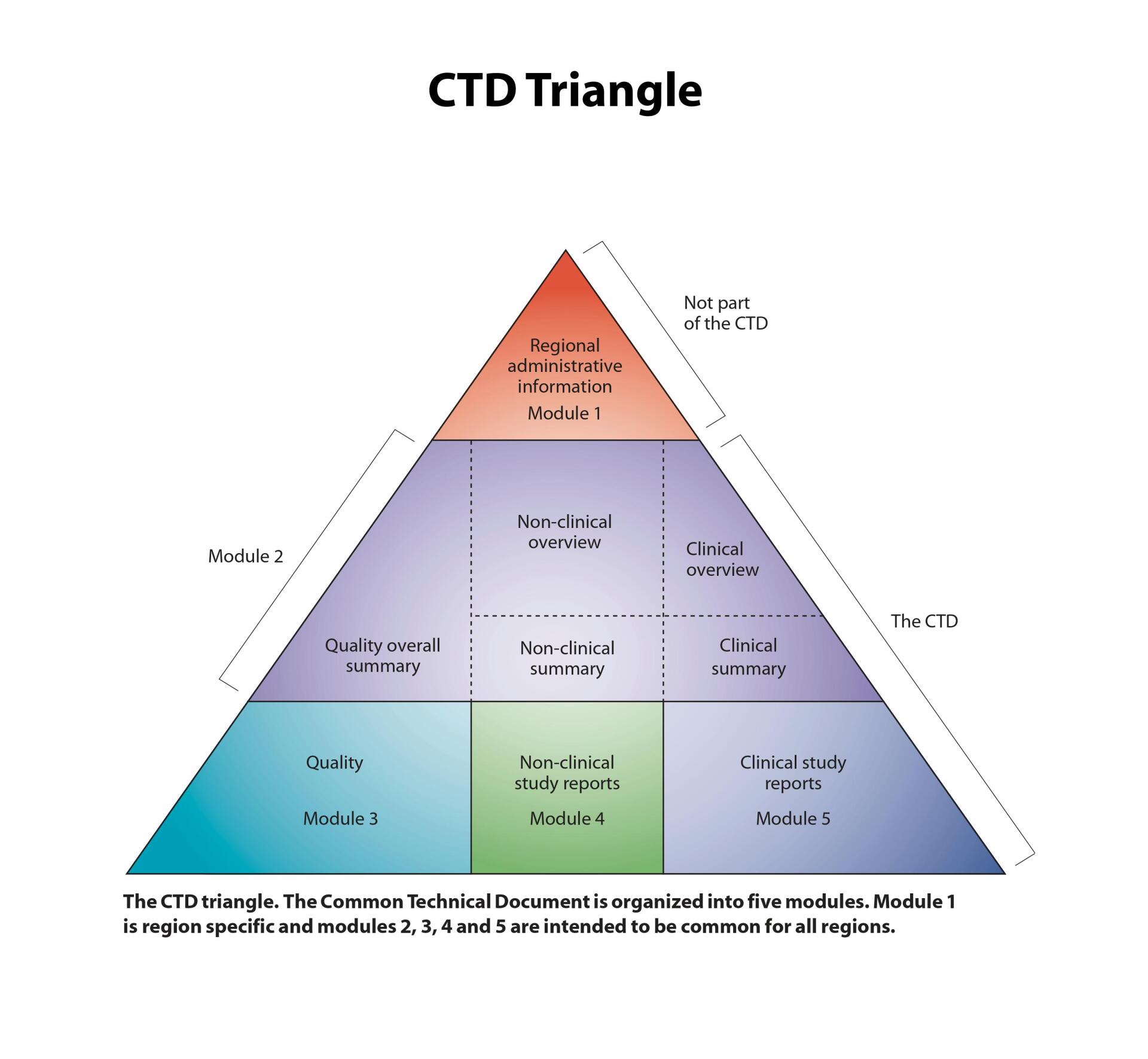
Data integrity has been a prime area of focus for regulatory agencies in recent years. A tremendous amount of data and information go into CMC reports and regulatory filings. We have significant experience in conducting Quality Control reviews of Analytical and CMC reports as well as Quality Control reviews of regulatory filings which include INDs and NDAs.